Central Venous Access Device (CVAD) Dressing Change Step-by-Step
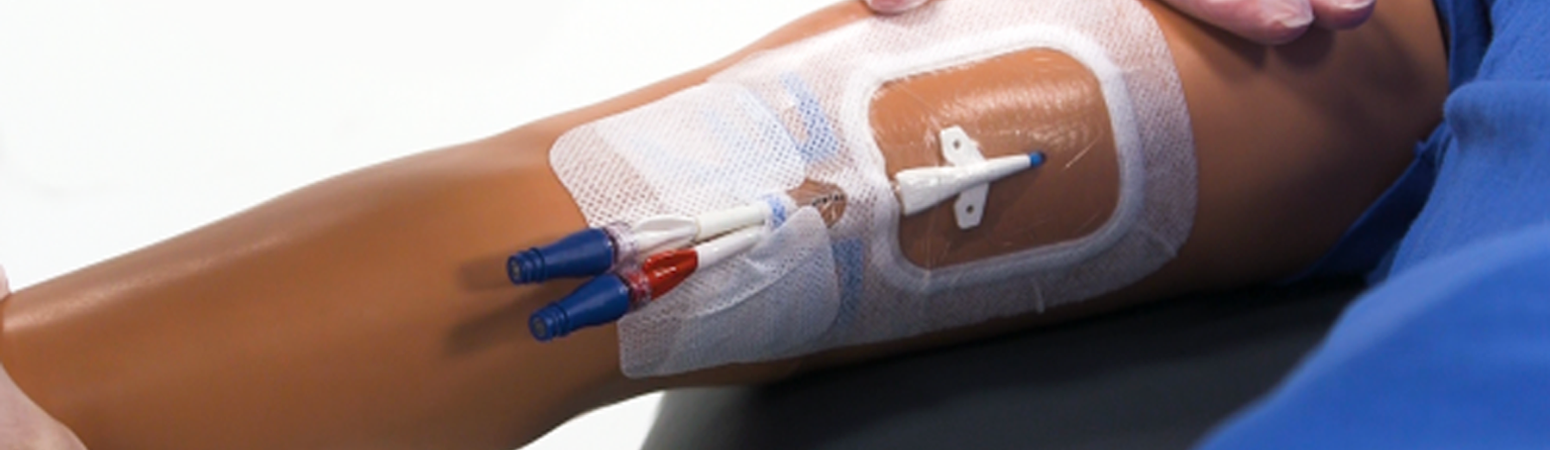
Central venous access devices (CVAD) require routine care and management, a process that involves assessment, removal of the dressing, skin antisepsis (i.e., site care), and dressing replacement. CVADs also require routine flushing and locking to maintain catheter patency. For some patients, blood may be withdrawn for laboratory studies.
A central vascular access device (CVAD) is commonly placed for patients who require weeks or months of infusion therapy. The most common type of CVAD is the peripherally inserted central catheter (PICC). Other types of CVADs include subcutaneously tunneled catheters, which are more common for very long courses of infusion therapy such as parenteral nutrition, and nontunneled CVADs, which are less often seen in home care. The implanted vascular port is another type of CVAD but is not addressed in this article.
In this article, we’ll review care and management of CVADs, the equipment and procedures for routine site care of CVADs, as well as procedures for maintaining CVAD patency.
For more information about different types of CVADs, refer to Home Infusion Therapy Part 1: Vascular Access Device Selection & Care.
This article was developed in alignment with the latest evidence-based research and the Infusion Nurses Society (INS) guidelines. All information has been reviewed by Lisa Gorski, RN, MS, HHCNS-BC, CRNI, FAAN, and Danielle Pierotti, RN, PhD, CENP, to ensure the content meets current professional guidelines and best evidence-based practices. The content of this article is taken directly from the MedBridge Clinical Procedure Manual, a comprehensive digital manual designed as a tablet-friendly resource for home health nurses and clinicians. The CPM is part of our broader home health software ecosystem aimed at providing efficient and effective onboarding and training solutions to home health agencies.
Enter your information below to download our Central Venous Access Device (CVAD): Site Care and Dressing Change step-by-step guide:
Email could not be subscribed.
Thank you for signing up!
Note: While this article presents evidence-based best practices, it is important to refer to your organization guidelines and/or specific physician orders regarding patient care.
How to Perform CVAD Site Care and Dressing Change
Before you begin CVAD care, talk to your patient, and determine a clean area where the patient can comfortably sit. Next, perform hand hygiene and prepare the field of supplies, then
open your CVAD kit and sterile barrier. Make sure to handle the sterile barrier by the edges. Additionally, before beginning, carefully pick up your mask and put it on. Now you’re ready to begin the CVAD site care and dressing change.
Step 1: Assess CVAD Insertion Site
- Put on your clean gloves.
- Carefully assess the insertion and the extremity for any signs of infection or evidence of venous thrombosis.
- Take note of any evidence of swelling, erythema, or drainage.
- Palpate the site gently to check for any tenderness.
- Remeasure the mid-arm circumference, and compare it to the baseline measurement.
Step 2: Remove the Current Dressing
Begin at the CVAD hub area, and gently remove the dressing perpendicular to the skin and toward the insertion site. Be careful not to dislodge the CVAD. If needed, you can secure the CVAD by taping the catheter outside of the dressing placement area.
Note the following:
- If present, remove the adhesive securement device, using alcohol to loosen the adhesive.
- A subcutaneous securement device is not removed.
- Tissue adhesive (TA) is replaced with each dressing change.
- If sutures are in place, assess their integrity.
Step 3: Prepare to Apply the New Dressing
Remove clean gloves, and perform hand hygiene. Put on sterile gloves.
Step 4: Perform the Skin Antisepsis
Apply alcohol-based chlorhexidine solution using a back-and-forth motion for at least 30 seconds. Allow to dry fully.
Step 5: Apply New Securement Device (if using)
Securement options include integrated securement dressings, tissue adhesive, adhesive securement devices, and subcutaneous securement systems. Apply skin barrier solution and allow to dry before placement of a new adhesive securement device.
Step 6: Apply TSM Dressing
Apply skin barrier solution to the area under the dressing, avoiding the area around the insertion site, and allow to dry fully. Then apply TSM dressing, making sure to apply without tension or stretching.
Note: an integrated securement dressing is used in this example.
Step 7: Label Dressing
Label the dressing with the date and your initials.
Site Care and Dressing Changes for Central Venous Access Devices (CVADs)
CVAD site care and dressing changes are performed at least every seven days when using a transparent dressing and every two days when using a gauze dressing. Note that the procedure for CVAD site care and dressing changes can also be applied to midline peripheral catheters. Midline catheters are generally in place for 2–4 weeks and will also require routine site care and dressing changes.
Key Measurements When Caring for a CVAD
- Measure the mid-arm circumference upon admission to home care as clinically indicated. An increase in circumference may be a sign of catheter-associated deep vein thrombosis.
- Measure the external length of the CVAD between the insertion site and the catheter hub to assess for evidence of CVAD dislodgment.
Equipment Considerations
- The preferred skin antiseptic agent is alcohol-based chlorhexidine solution. If a patient is allergic to chlorhexidine, 70% alcohol (without chlorhexidine) or povidone iodine may be used instead.
- The use of skin barrier solutions reduces the risk of skin injury around the CVAD.
- CVAD securement is an important aspect of care and management as it reduces the risk for inadvertent dislodgement and catheter pistoning at the insertion site. Options for securement include
- Integrated securement dressing: the dressing has securement properties as listed by the manufacturer
- Tissue adhesive: two to three drops of “glue” at the catheter insertion site—can also be placed under the catheter hub or wings
- Adhesive securement device: an adhesive pad under the dressing to which the catheter is secured
- Subcutaneous securement system: posts that hold the catheter in place subcutaneously and stay in place for the duration of catheter dwell
Dressing Considerations
- Allow antiseptic solution to dry fully before applying transparent semipermeable membrane (TSM) dressing. Applying a TSM dressing to damp skin increases the risk for a skin reaction under the dressing.
- Chlorhexidine-impregnated dressings (foam or gel) may be used with some patients at increased risk for infection or in accordance with an organization’s policy, although there is a lack of evidence regarding the efficacy for long-term use.
- For some patients, there may be challenges in maintaining the TSM dressing, such as diaphoresis. For these patients, additional liquid adhesive, such as gum mastic, may be used to enhance adhesiveness of the TSM dressing by applying it under the edges of the dressing. Use a skin barrier solution under the adhesive and remove carefully to prevent skin damage—an adhesive remover is suggested.
- If a patient has a sensitivity or allergic reaction to a TSM dressing, a gauze dressing can be used in its place. However, changing to a different brand of TSM dressing is also considered because TSM dressings are made of different materials.
Tips for Flushing and Locking Central Venous Access Devices (CVADs)
- CVADs are flushed and aspirated for a blood return prior to each infusion to assess catheter function. A blood return is defined as blood that is the color and consistency of whole blood.
- CVADs are flushed with saline after each infusion to clear the infused medication from the catheter lumen and are locked after completion of the final saline flush to decrease the risk of occlusion. Both saline and low-concentration heparin (e.g., 10 units/ml) may be used for CVAD locking.
- Prefilled saline and heparin syringes are used. Saline should not be withdrawn from IV bags as a flushing solution due to risk for contamination and infection.
- Note that the procedure for CVAD flushing and locking can also be applied to midline peripheral catheters.
CVAD Precautions
- If unable to obtain a blood return or flush the CVAD, assess for possible occlusion and potential use of a thrombolytic medication.
- If there is any evidence of infection or pain associated with the CVAD, report it to the provider.
Note: While this article presents evidence-based best practices, it is important to refer to your organization guidelines and/or specific physician orders regarding patient care.
Home Health Software Solution
This CVAD step-by-step is just one small piece of our full Clinical Procedure Manual for home health. Our CPM is a tablet and mobile-first solution to assist home health nurses and aides in the field, at the point of care. CPM is part of our greater home health software solution, which includes a skills and competency manager, learning management system, and full onboarding and training curriculums.




